How to prepare 0.025N Silver Nitrate?
To prepare 0.025N Silver Nitrate, need to know molecular mass of Silver Nitrate.
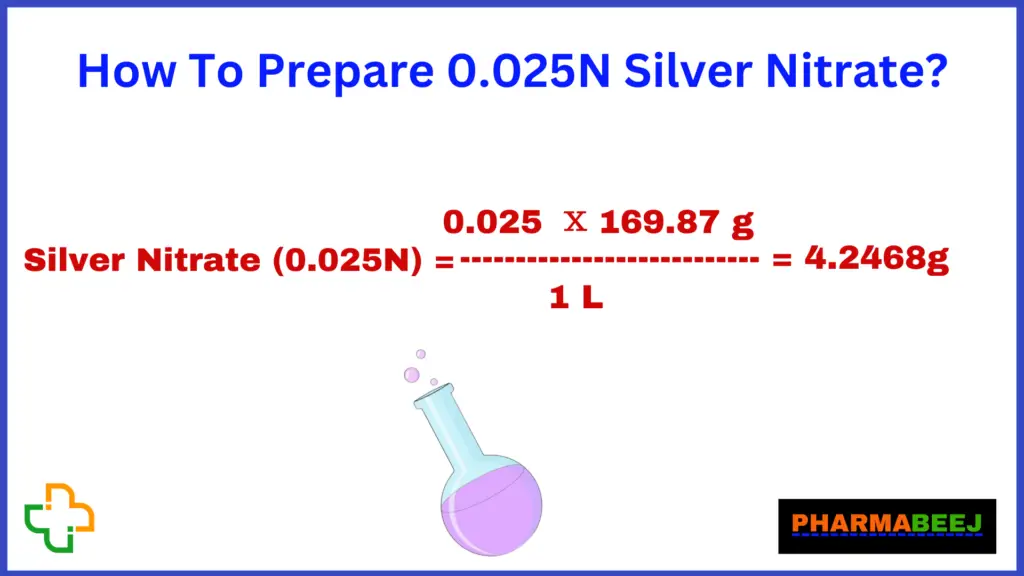
The molecular mass of Silver Nitrate is 169.87 gm/mol.
Calculation Formula for 0.025N Silver Nitrate:
Calculate the amount of Silver Nitrate required for 1L of solution by formula mentioned below;
Required Amount of Silver Nitrate = 0.025N x 1 L x 169.87 = 4.2468 g
Preparation of 0.025N Silver Nitrate:
- Weigh 4.2468 g of Silver Nitrate.
- Transfer the weighed amount into 1000ml of volumetric flask.
- Add about 500ml of distilled water into the volumetric flask.
- Dissolve the weighed amount of silver nitrate.
- Dilute up to the mark to 1000ml with distilled water.
Also Read:
- How to prepare 0.1N HCl in pharma?
- Preparation and standardization of 1N NaOH
- Preparation of 0.1M Lead Nitrate In Pharma
- Preparation of 0.05M Disodium Edetate VS
- Iodine Volumetric Solution Preparation and Standardization
- Understand 14 ICH guidelines in short
- What is Phase 0 clinical trial in pharma?
- Dissolution calibration in pharma
- Karl Fischer Titration Interview Question and Answer
- 4 Different Types of titrations
Refer YT Channel: Pharmabeejpro