Procedure for Preparation And Standardization of 1N NaOH:
Reagents used for 1N Sodium Hydroxide:
- 95% Alcohol
- Phenolphthalein Indicator
- Carbon Dioxide Free water
Preparation of Reagent:
95% Alcohol: Mix 95% of absolute alcohol with 5ml of water.
Phenolphthalein Indicator: Dissolve 1 g of Phenolphthalein in 100ml of 95% alcohol.
Carbon Dioxide free water: The water which has been boiled vigorously for a 5 min or more and allow to cool protected from the adsorption of carbon dioxide from the atmosphere or purified water that has a resistivity of NLT 18 Mohm-cm.
Preparation of 1N Sodium Hydroxide (1N NaOH):
- Take 200ml dried beaker.
- Add about 162g of sodium hydroxide in 150 ml of carbon-dioxide free water.
- Dissolve it and cool the solution to room temperature.
- Fiilter the soolution through hardened filter paper.
- Transfer 54.5 ml of filtrate to a tight, polyolefin container.
- Dilute the solution with water to 1000 ml.
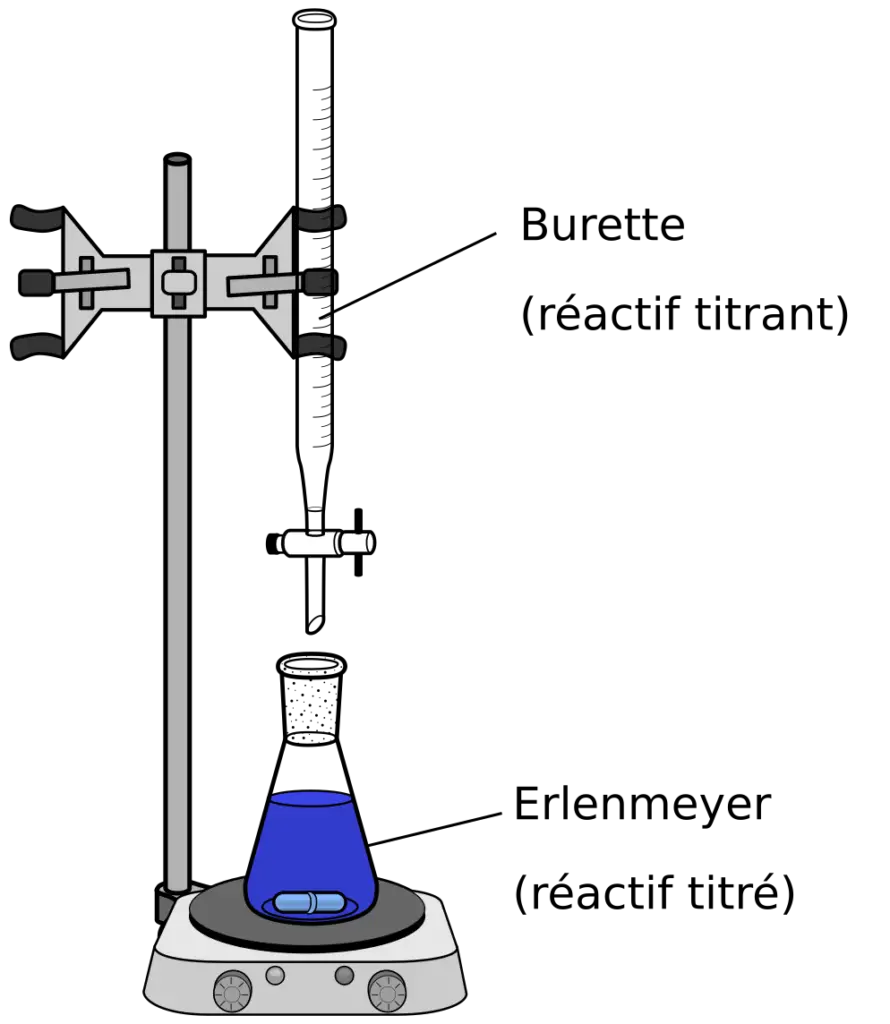
Standardization of 1N Sodium Hydroxide (1N NaOH):
- Weight accurately 5g of potassium bi-phthalate (potassium hydrogen phthalate), previously crushed and dried at 120°C.
- Dissolve it in 75ml of carbon di-oxide free water.
- Add 2 drops of phenolphthalein indicator solution.
- Titrate the solution with 1N sodium hydroxide to the production of permanent pink color.
Calculation of Normality of Sodium Hydroxide:
Normality of Sodium Hydroxide = W/0.20422 x V
Where,
W= Weight of potassium hydrogen phthalate in g,
V= Volume of Sodium Hydroxide consumed in ml.
This is the procedure for Preparation And Standardization of 1N NaOH in pharmaceuticals.
Also Read:
- How to prepare 0.1M HCl Solution
- Laboratory Investigation of aberrant results part-3
- Mechanical Calibration of Dissolution
- Difference Between Alcoa and Alcoa Plus
- Practices to avoid data integrity issues and alcoa plus
- Interview Questions and answers for QC
For interview preparation refer
YT channel: Pharmabeej