Iodine Volumetric Solution Preparation and Standardization (0.1 N) as per USP:
Preparation of Reagent:
Starch TS: Mix 1 gm of soluble starch with 10 mg of red mercuric iodide and a sufficient amount of cold water to make a thin paste. Add 2 ml of boiling water, and boil for 1 min with continuous stirring. Cool, and use only a clear solution.
1N Hydrochloric Acid: Dilute 85ml of Hydrochloric acid into 1000ml of water.
Sodium Thiosulfate VS (0.1 N): Dissolve about 26gm of sodium thiosulfate and 200mg of sodium carbonate in 1000ml of recently boiled and cooled water.
Iodine Volumetric Solution Preparation (0.1 N):
Dissolve about 14gm of Iodine in a solution of 36gm of Potassium Iodide in 100ml of water, add 3 drops of Hydrochloric acid, and dilute with water to 1000 ml.
For lower strength refer to the given table:
| Strength of VS | Quantity of Iodine to be taken (g) | Quantity of Potassium Iodide to be taken (g) | Makeup with water (ml) |
| 0.01 N | 1.4 | 3.6 | 1000 |
| 0.05 N | 6.5 | 18 | 1000 |
Standardization of Iodine Volumetric Solution (0.1 N):
- Transfer 25ml of the Iodine solution into a 250ml of volumetric flask.
- Dilute to volume with water to 100ml.
- Add 1ml of 1N hydrochloric acid.
- Swirl gently to mix.
- Titrate with 0.1 N Sodium thiosulfate VS until the solution has a pale yellow color.
- Add 2 ml of starch TS.
- Continue the titration until the solution turns colorless.
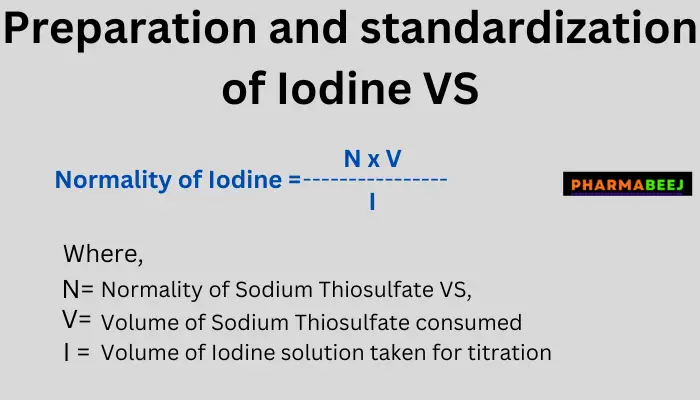
Calculation formula of Iodine Volumetric Solution (0.1N):
- N x V
- Normality of Iodine = —————
- I
Where,
- N= Normality of Sodium Thiosulfate VS,
- V= Volume of Sodium Thiosulfate consumed,
- I= Volume of Iodine solution taken for titration
Also Read:
- Understand 14 ICH guidelines in short
- What is Phase 1 clinical trial in pharma?
- Why is Data integrity important in pharma?
- How to prepare 0.1N HCl in pharma?
- Preparation and standardization of 1N NaOH as per USP
Refer YT Channel: Pharmabeejpro