Molarity of 37% HCl Solution
Hydrochloric Acid is nothing but the hydrogen chloride gas aqueous solution. The molar mass of hydrochloric acid is 36.6 g/mol and the density of 37% hydrochloric acid is 1.2 g/ml.
Calculate the weight of hydrochloric acid for 1000 ml, using the mentioned formula.
Weight = volume x density
Weight = 1000 x 1.2 = 1200 g
So, the weight of 1000 ml of hydrochloric acid is 1200g.
Calculating the amount of hydrogen chloride in the 1200g based on this information.
100 g of hydrochloric acid contains 37 g of hydrogen chloride.
So, 1 g of hydrochloric acid have 0.37 g (37/100) of hydrogen chloride.
1200 g hydrochloric acid having 1200 x 0.37 = 444 g of hydrogen chloride.
According to the calculations described above, the 1000 ml of hydrochloric acid solution contains 444 g of hydrogen chloride.
Then the molarity of 37% HCl is,
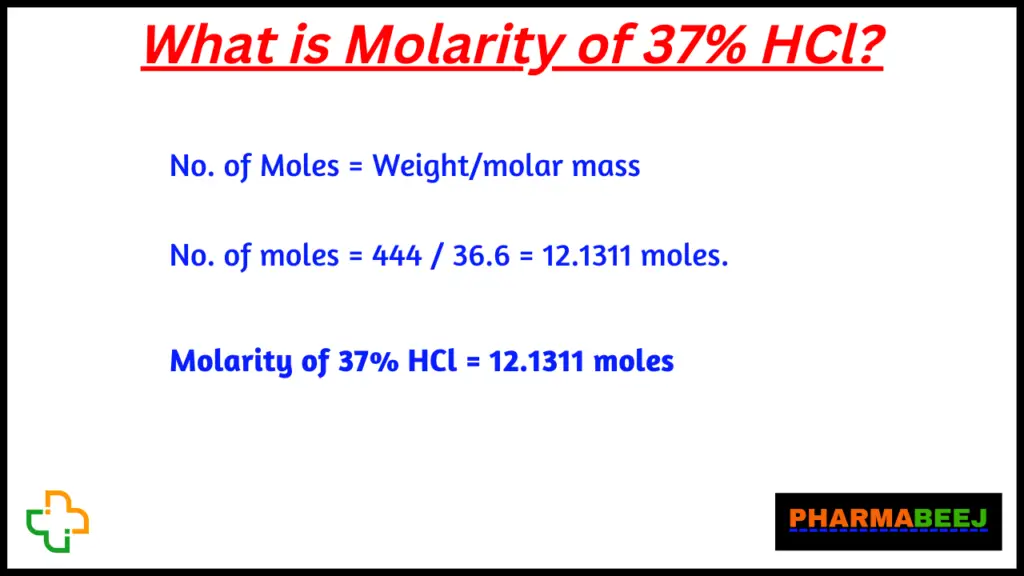
No. of Moles = Weight/molar mass
No. of moles = 444 / 36.6 = 12.1311 moles.
What is Molarity of 37% HCl?
Also Read:
- How to prepare 0.1N HCl in pharma?
- How to prepare 0.5M HCl Solution?
- Preparation and standardization of 1N NaOH
- How to prepare 0.1N Silver Nitrate in Pharma?
- What are GAMP 5 software categories
- What is Phase 0 clinical trial in pharma?
- HPLC Calibration Parameters in pharma
- HPLC Interview Question and Answers
- QA Interview Q&A
- FAQ on FDA’s Data integrity
Refer YT Channel: Pharmabeejpro