What is molality in pharmaceuticals?
Molality is nothing but the ratio of moles of solute to the kilogram of solvent.
or
molality (m) is a unit of concentration that measures the number of moles of solute per kilogram of solvent. It is different from molarity, which expresses the concentration in terms of moles of solute per liter of solution. Molality is often used in certain situations where changes in temperature or volume are not significant or when the density of the solvent may change.
The moles of solute refer to the amount of the substance being dissolved, and the mass of the solvent is expressed in kilograms.
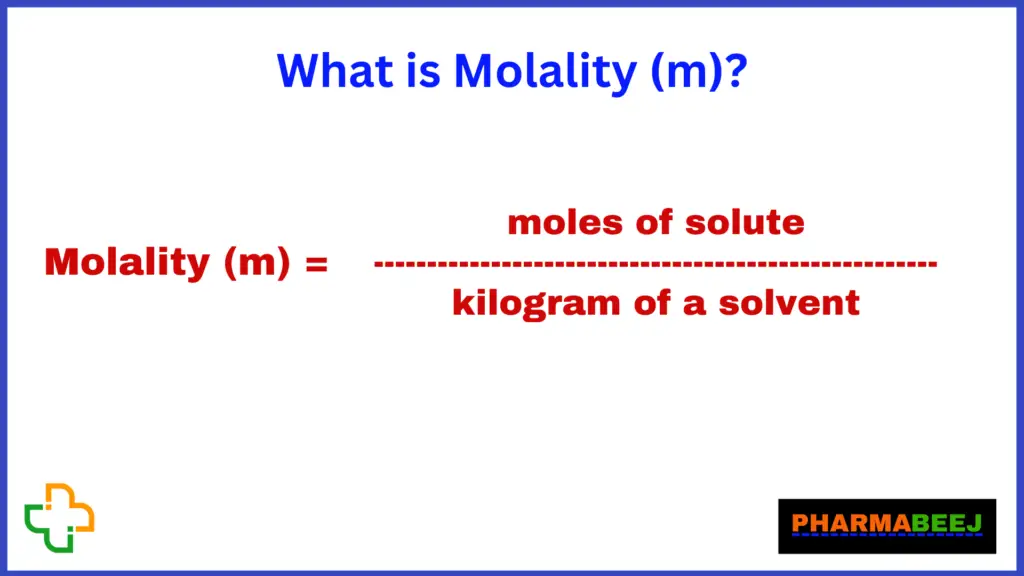
What is Molality?
Also read:
Interview Q&A:—–>
- Karl Fischer Titration Interview Question and Answer
- Clinical Data Management Interview Questions And Answers
Calibration:—–>
Article:—–>
- Understand 14 ICH guidelines in short
- What is data integrity and Alcoa plus?
- What is Phase 1 clinical trial in pharma?
- Preparation of 1N Ethanolic Potassium Hydroxide (KOH)
- How to prepare 0.1N HCl in pharma?
- What is Difference between assay, potency and purity?
Refer YT Channel: Pharmabeejpro