Procedure for Loss On Drying USP test:
- Take a dry LOD bottle as specified as per standard operating procedure (SOP).
- Place the empty LOD bottle in the oven at a given temperature for 30 mins or specified in standsrd testing procedure (STP).
- Cool the empty LOD bottle in the desiccator at room temperature for 30min.
- Take a weight of empty LOD bottle (W1).
- Add evenly distrubuted 1 to 2 gm of the sample or specified as per STP and weigh the LOD bottle (W2).
- Place the LOD bottle in the oven at a given temperature for 1 hr or specified as per STP. (Bottle cover shall be open.)
- Maintain the oven temperature throughout the 1 hr or at a specified temperature.
- Remove out the LOD bottle from the oven and allow it to room temperature in a desiccator for 30 mins.
- Reweigh the LOD bottle after cooling (W3).
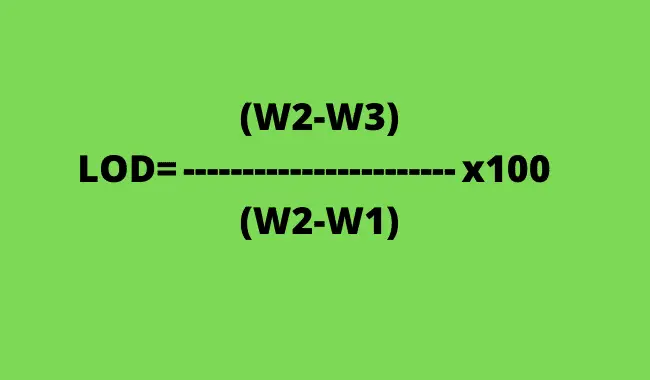
How to calculate the Loss on Drying (LOD) as per USP:
Here is the formula for calculation of LOD as per USP;
Where,
W1= Empty LOD bottle weight
W2= Sample + LOD bottle
W3=Weight after drying
How to perform LOD up to constant weight?
If the test to be perform up to constant weight, then need to perform same as mentioned above and further below mentioned procedure.
- Place LOD bottle in oven for 1 hr at a given temperature.
- Remove LOD bottle from oven.
- Allow to cool it to room temperature in desiccator.
- Weigh the LOD bottle (W4)
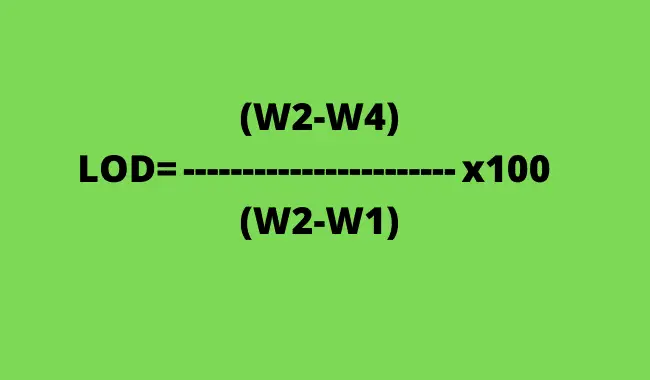
How to calculate LOD constant weight?
Calculation formula for loss on drying usp for constant weight;
W4= Weight after drying for constant weight.
As per USP the acceptance criteria for constant weight is; the difference between 2 consucative weight should be not more than 5mg per gm.
Also read:
What is Clinical Trial as per FDA
HPLC Interview Question and Answers
Gas Chromatography Interview questions and answers
Karl Fischer Titration Interview Question and Answer
Dissolution Interview Question and Answer
What Is Change Control In pharma?
CAPA Process in Pharmaceutical Management System
Interview Question and answer on Polarimeter
For interview preparation refer
YT channel: Pharmabeej