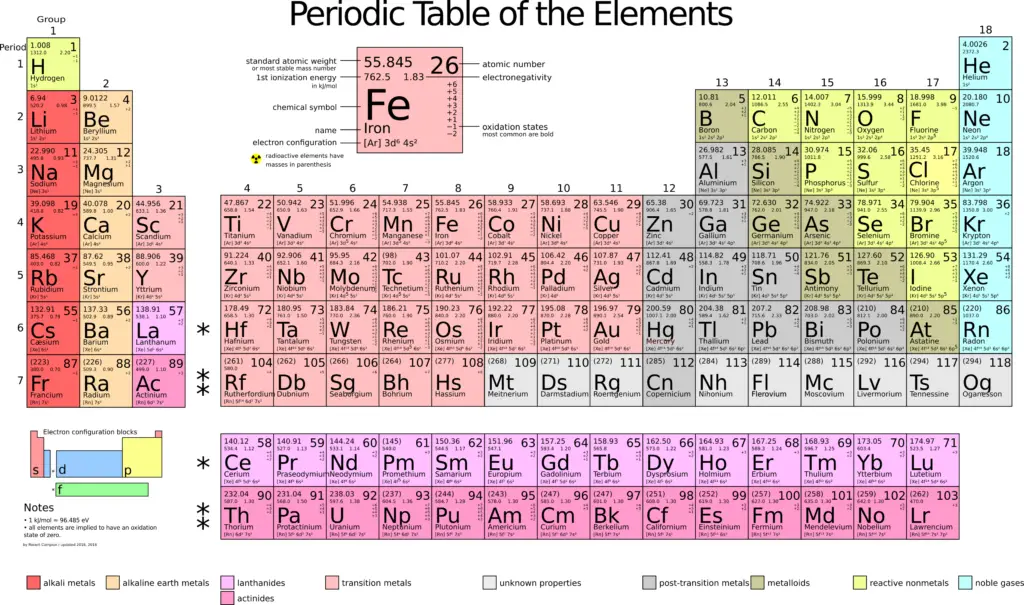
Periodic Table Of Elements:
The periodic table, also known as the periodic table of chemical elements, represents the chemical elements in tabular form. The periodic table is widely used in chemistry, physics, and various other sciences, and has generally been considered an icon of chemistry.
Atomic mass (or daltons, D) is the average mass of each atom of an element measured in atomic mass units (amu). The atomic mass of an element is determined by a weighted average of the masses of all its isotopes multiplied by their abundances. Atomic mass is also known as atomic weight, although the word “mass” is more accurate.
When combined with the mole concept, the atomic mass is useful in chemistry: an atomic mass expressed in amu is equivalent to the mass expressed in grams of one mole of an element.
Because one mole of iron atoms weighs 55.847 grams, one mole of iron would weigh 55.847 grams. This calculation can also be performed for molecules and ionic compounds.
In his first periodic table of the elements, Dimitri Mendeleev arranged the elements by increasing atomic weight, since the nucleus of the atom and its interior structure were unknown at the time.
Instead of being arranged by increasing atomic weight, the modern periodic table is arranged by increasing atomic number.
In the periodic table of elements, the rows are called periods, while the columns are called groups.
Also Read:
- Understand 14 ICH guidelines in short
- What is form 482-483 and 484
- Electronic Code of Federal Regulation (E-CFR)
- Clinical Data Management Interview Questions And Answers
For interview preparation refer
YT channel: Pharmabeej