IR Spectroscopy principle and Application:
 |
| IR spectrometer |
While surviving in day-to-day life we all are surrounded by various particles, Waves, rays, radiations which we can not see by the naked eyes. Infra-Red is one of the radiations available in the surrounding which continuously emits from the Sun.
- Background of Infra-red (IR)
- Principle of IR spectroscopy
- Electromagnetic spectrum range of IR
- What is IR spectroscopy?
- Application of IR spectroscopy
- Is IR radiation are Harmful
- Difference between IR and FTIR
1. Background of Infra-red (IR):
Here you can see the experiment of Herschel to measure the temperature of different colors through a thermometer.
The Infrared spectroscopy is a vibrational energy level changes when radiation passes through the material.
The infrared spectroscopy is also known as vibrational spectroscopy. Infrared spectroscopy is based on the absorption or transmission of radiation.
3. Electromagnetic spectrum range of IR:
- Near IR ranges from 14000cm-1 to 4000cm-1 (0.7- 2.5 micrometer)
- Mid IR ranges from 4000cm-1 to 400cm-1( 2.5 – 25 micrometer)
- Far IR ranges from 400cm-1 to 0cm-1 (25 – 1000 micrometer)
Radio waves–> Which gives us instant communication eg. Mobile tower.
Microwaves-> Data and Heat eg. Microwave oven.
Infrared-> It gives invisible heat eg. TV remote light.
Visible light-> The light which we can see. eg Rainbow.
Ultraviolet light-> Energetic Light eg. Sun.
X-ray-> Penetrating Radiations eg. X-Ray photos.
Gamma rays-> Can destroy living cells, Nuclear energy eg. Citi scan.
4. What is IR spectroscopy:
IR spectroscopy is a common technique to identify the functional group of the material. This technique is based on the absorption, emission, or transmission of light. When IR rays passed through the material some of the radiation is absorbed by the material, some is reflected, and some are transmitted from the material. When rays are passed through the material it absorbs rays and produces a sharp valley and when rays are transferred through the material it produces a straight line which means when the valley produces it indicates vibrational energy level changes.
There are two major types of vibrations :
1 Stretching and 2. Bending vibration
1. Stretching vibration:
There are two types of stretching vibration: i. Symmetric vibration ii. Asymmetric vibration.
i. Symmetric vibration:
 Symmetric vibration |
ii. Asymmetric vibration:
In asymmetric vibration one bond is vibrated shorten and the other is longer. Means one molecule comes towards the central atom while the other is go away from a central atom in the same direction. As shown in the image.
 |
| Asymmetric vibration |
2. Bending vibration:
It is a variation in the angle of bond cause due to vibration.
There are four types of bending vibration:
i. Scissoring, ii. Wagging, iii Rocking, iv.Twisting
Scissoring and rocking are in a plane vibration and wagging and twisting are out of plane vibration.
i. Scissoring vibration:
This is in-plane vibration. In this vibration, both molecules will be on the same distance from the atom but both molecules will come close to each other and away from each other. Eg. as scissors. Just have a look at the shown image.
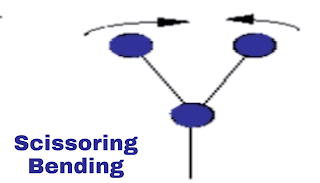 |
| Scissoring vibration |
ii. Rocking vibration:
This is also a plane vibration. In this vibration both the molecule are on equal distance from atom and both are swing in a plane direction and back in same direction. Here in image you can see.
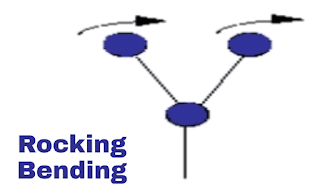 |
| Rocking vibration |
iii. Wagging vibration:
This is out of plane vibration. In this vibration both the molecule will be on same distance from atom but both will move together out of plane from the atom. As shown in the image.
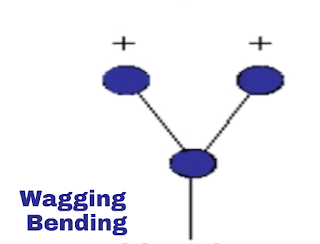 |
| Wagging vibration |
iv. Twisting vibration:
This is also out of plane vibration. In this vibration, molecules are moving out of a plane from atom but not in together. Refer to the below-shown images.
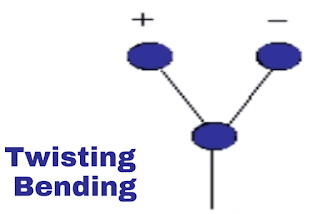 |
| Twisting vibration |
Does Infrared have color?
The infrared is out of visible spectromagnetic range, it does not having any colour but by applying certain artificial condition it can be visible.
The TV remote is the best day to day life example. Press any button of remote and hold the mobile camera in front of the TV remote light. Impression can be seen on the mobile display. Here is an image of infrared.
 |
| IR image of Human Being |
Region in IR (Mid IR) spectroscopy:
Two types of the region in IR spectroscopy i.Functional group region ii. Fingerprint region
i. Functional group region identifies individual peak and it is easy to identify the peak in this region. ( Equal or greater than 1500 cm-1)
ii. Fingerprint region is having multiple peaks so it is complicated to identify peaks in this region. ( Less than 1500cm-1)
Calculation of wavelength or wavenumber:
The calculation of wavelength or wavenumber can be done by this equation.
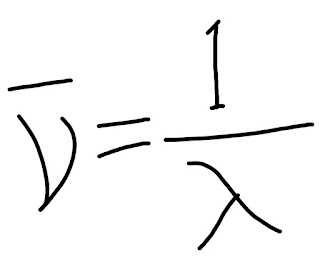 |
| Wavenumber calculation equation |
neu= denotes wave number
Lambda= denotes wavelength
There is a relation between the wavelength and wavenumber, as per the above equation
Wave number is inversely proportional to the wavelength.
wavenumber can calculate if the wavelength is known.
And the unit of measurement is cm-1.
Why KBr is used in IR and not water?
In IR spectroscopy water shows two strong peaks and water is a strong polar solvent, due to this reason water is not suitable for IR spectroscopy.
Lamp Used in IR Spectroscopy:
In IR spectroscopy three types of lamp can be used as per their scanning range
For Near IR Tungsten halogen lamp
For Mid-IR Nernst flower or Globar
For Far IR High-pressure mercury lamp
5. Application of IR spectroscopy:
- IR spectroscopy is mainly useful for organic and inorganic chemistry.
- IR spectroscopy is used in the pharmaceutical industry for functional group identification.
- Useful for food analysis.
- Useful for petrochemical engineering
- Useful for the polymer industry.
- Nanoscale semiconductor
- Space exploration
6. Is IR radiation are harmful:
Yes, IR radiation is harmful to a human being if continuously come in contact with skin. It damages to tissues and may cause skin cancer. It impacts on eye lens or cornea.
7. Difference between IR and FTIR:
1. IR stands for infrared and FTIR standard for Fourier transform infrared.
3. IR is a slow and imprecise method while FTIR is a fast and precise method as compared to IR.
4. IR having fix mirror which produces only constructive interference while FTIR having two mirrors ( Fix and Moving) which can produce constructive as well as destructive interference.
Additional information:
What is Constructive interference?
The FTIR is having two mirrors one is fixed and the other is a moving mirror. Both the mirrors on equal path distance. When infrared light hits the beam splitter, the splitter splits the rays. some rays are transferred towards fix mirror and some reflect towards moving mirrors and when rays come back from mirrors to beam splitter they make the same path difference with equal time and forms constructive interference.
 |
| FTIR |
What is destructive interference?
When rays are splits from the beam splitter, transferred towards the fixed mirror and reflected towards the moving mirror. But here the moving mirror moves back and makes more distance than fix mirror. when rays come back from mirrors to the beam splitter, the rays from moving mirrors take more time and they make path difference and time variation in such case they form destructive interference.
Conclusion:
In this article, we learn about what is IR Spectroscopy, IR spectroscopy Principle and application, types of IR, Electromagnetic range of IR Spectroscopy.
- Reference:
USP general chapter 854 represents IR spectroscopy ( Mid IR)
Refer YT Channel: Pharmabeej
Also read:
X-Ray diffraction
ICH Quality guideline in Shortcut - GC Interview question and answers
- Quality Control Interview Question and answers
Thanks for Reading IR spectroscopy principle and application article
Sharing is pairing to Loved…!!!
