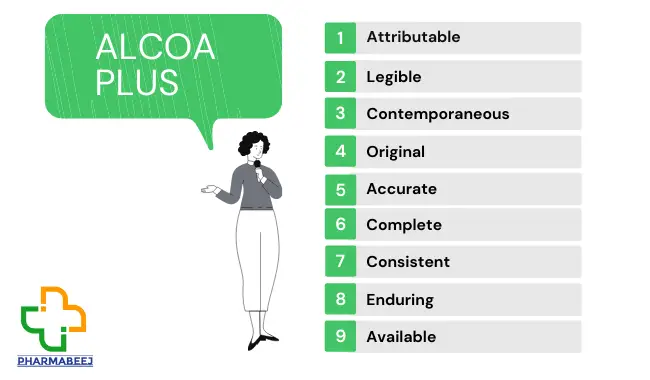
Definition of ALCOA Plus:
1. A-Attributable:
Attributable means recording who performed an action and when. And, if a record is changed, who did that and why.
2. L-Legible:
Legible means that the data must be recorded permanently in a durable medium and should be readable.
3. C-Contemporaneous:
Contemporaneous means that the data should be recorded at the time the work is performed, and the date and time stamps should follow.
4. O-Original:
Original data includes the first capture of data or information and all subsequent data required to fully reconstruct the conduct of the GMP activity.
5. A-Accurate:
Accurate means data collected is correct, truthful, complete, valid and reliable.
6. C-Complete:
Complete is the data must be whole i.e. complete set including repetitions.
7. C-Consistent:
Consistent application of data timestamps in the expected sequence.
8. E-Enduring:
Enduring is durable. i.e.lasting throughout the data lifecycle.
9. A-Available:
Available is readily accessible for review or audit for the lifetime of the record.
Also Read:
Best Practices to Avoid Data Integrity.
Annual Product Quality Review (APQR) in pharma
For interview preparation refer
YT channel: Pharmabeej