Data, records and their properties:
Raw Data: Raw data (Source data) is the original record (Data) which can be described as the first captured of information, whether recorded on paper or electronically.
Raw data permits full reconstruction of the activities.
Static Data: A static data record format is one that is fixed and allows no interaction between the user and record content e.g. paper record or electronic image.
In this, once records are created, the output cannot be changed.
Dynamic Data: Interaction between the user and the record content. e.g. a chromatographic record may allow the user to change the baseline and reprocess chromatographic data so that the resulting peaks may appear smaller or larger.
Meta Data: Metadata are data that describe the attributes of data such as the structure, data elements and inter-relationships e.g. audit trails.
Metadata provides context and managing to data. Without the context provided by metadata, the data has no meaning.
Metadata form an integral part of the original record.
Metadata is described as data about data.
E.g. weigh 3.5 gm NaOH, in this sentence bold (gm) represents data and italic words represent metadata.
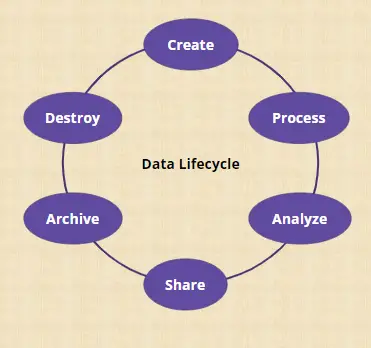
Data lifecycle: All phases in the life of data from generation and recording through processing (including analysis and transformation), sharing and usage, data retention, archive/retrieval and destruction.
Also Read:
- What is data integrity and Alcoa plus?
- What is the Difference between assay, potency and purity?
- 10 principles of Current Good Manufacturing Practices
- Why Data integrity important in pharma?
- CAPA process in the pharmaceutical management system
- Best Practices to avoid data integrity issues Alcoa plus
- The practices with data integrity risk (DI Risk)
- Karl Fischer Titration Interview Question and Answer
- HPLC Interview Question and Answers
- HPLC Calibration Parameters in Pharma
Refer YT Channel: Pharmabeejpro