Content Uniformity Acceptance Limit:
Content Uniformity is one of the test to measure that drug content is uniformly available in each tablet or capsule.
While the blending stage the drug is mixed with other raw materials. To identify whether the drug content mixed uniformly content uniformity test is performed.
How to Perform Content Uniformity test:
Content Uniformity test can be performed in 2 stages such as L1 and L2.
- Content uniformity is performed on 10 units.
- Take the flask mentioned in the STP.
- Add 1 unit in each flask.
- Perform analysis as mentioned in STP.
- The obtained results should be within the acceptance criteria.
- The passing criteria is, the acceptance value should be not more than 15.
Calculation Formula of Acceptance Value:
The content uniformity acceptance limit can be calculated by using this formula:
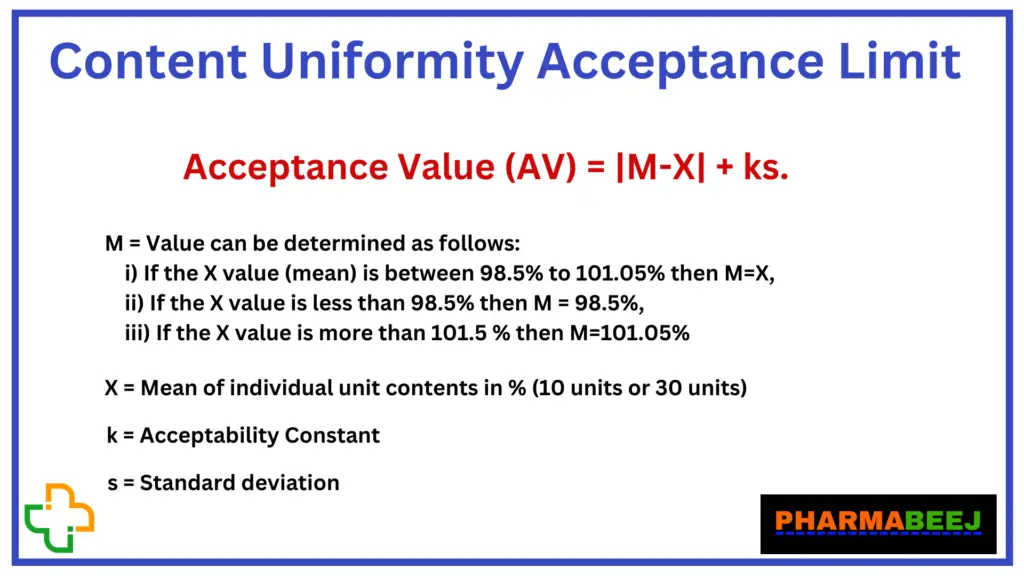
Acceptance Value (AV) = |M-X| + ks.
Where,
X = Mean of individual unit contents in % (10 units or 30 units)
k = Acceptability Constant
If the number of units tested is 10, then k=2.4
If the number of units tested is 30, then k=2.0
s = Standard deviation
M = Value can be determined as follows:
i) If the X value (mean) is between 98.5% to 101.05% then M=X,
ii) If the X value is less than 98.5% then M = 98.5%,
iii) If the X value is more than 101.5 % then M=101.05%
The acceptance value should be not more than 15 (L1 = 15).
What if the L1 stage fails?
If the L1 stage is not meeting the acceptance criteria then the L2 stage shall be executed.
In the L2 stage, the next 20 units shall be analyzed and the acceptance value shall be calculated.
The content uniformity acceptance limit is:
The acceptance value should be not more than 15 for 30 units of the L1 + L2 stage, and no unit shall be less than ( 1 – L2 x 0.01) M and not more than ( 1 + L2 x 0.01) M. In this formula L2 = 25.
Also Read:
- Preparation and standardization of 1N NaOH as per USP
- Best Practices to avoid data integrity issues alcoa plus
- Principle of Quality control instruments
- What is a clinical trial in pharma?
- What is UPLC Principle in pharma?
Refer YT Channel: Pharmabeejpro