What is Computer system validation?
Food and Drug Administration (FDA) guidelines for software validation outlines general validation principles applicable to medical device software or software used to design, develop, or manufacture medical products.
“A computerized system validation process (also called a “Computer System Validation”) is the process of testing/validating/qualifying a computerized system under a regulated standard (e.g. FDA 21 CFR part 11) to ensure that it performs as intended in a safe, secure and reliable way.”
Refer General Principles of Software Validation; Final Guidance for Industry and FDA Staff
According to 21 CFR Part 11, companies must follow good business practices. An organization can implement computer systems under Part 11 that will greatly enhance individual performance, reduce errors by identifying risks, and increase overall productivity.
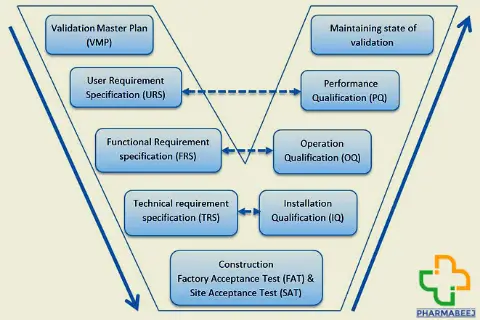
A computer system validation protocol can be written by following the steps below.
- User Requirement Specification
- Design Qualification
- Installation Qualification
- Operation Qualification
User Requirement Specification:
The URS is prepared for the intended system and describes the critical functionalities that are needed for our analysis.
Design Qualification:
In a design qualification, we ensure that the computer system we purchase is in line with the user requirements, as well as being able to run the instrument that will be connected to it.
Installation Qualification:
The following steps should be followed during installation qualification:
- Compare the computer system and its components with the purchase order.
- Make sure all necessary documents are checked, such as manuals, safety and validation certificates, and maintenance instructions.
- Look for any damage that occurred during shipping.
- By connecting its components, install the system.
- Turn on the system and check if all parts and components are working. Perform an electronic self-test.
- Follow the manufacturer’s instructions to install the software.
- Install the correct software.
- Configure equipment modules, including printers.
- Record all hardware and software installed on a computer along with their descriptions.
- The manuals and SOPs for the equipment should be listed.
- To define all serial and revision numbers of the hardware and software, an installation report should be prepared.
- Among the information provided in the report should be the size of the hard drive and RAM, the version of the operating software, the serial number of the monitor, the serial number of the printer, the manufacturer, and the type of connection.
Operational Qualification:
There are two things to computer system validation, one is the computer and the other is the instrument software. The instrument software should be qualified separately from the computer.
May this will help you to understand the computer system validation in pharmaceutical industry.
Frequently Asked Questions:
- Q: Should my computer system be validated?
- A: Yes, the computer system is used to provide the information to the regulatory agencies or to meet the regulatory requirement the computer system should be validated.
- Q: Should third-party software used for routine analysis be validated?
- A: Yes, the software which is used to produce results or data should be validated.
- Q: Should Excel used for calculation be validated?
- A: Yes, Excel is used to calculate the results.
Also Read:
- Understand the 14 ICH guidelines
- OOS Investigation Checkpoints
- Requirement for conducting stability study
- How to prepare 0.1N Hcl solution
- What is Data integrity and Alcoa Plus
- Difference between assay, potency and purity
- Karl Fischer interview question and answers
Refer YT Channel: Pharmabeej